Brain-Chip Implants Retracted: Elon Musk’s Neuralink Suffers Setback
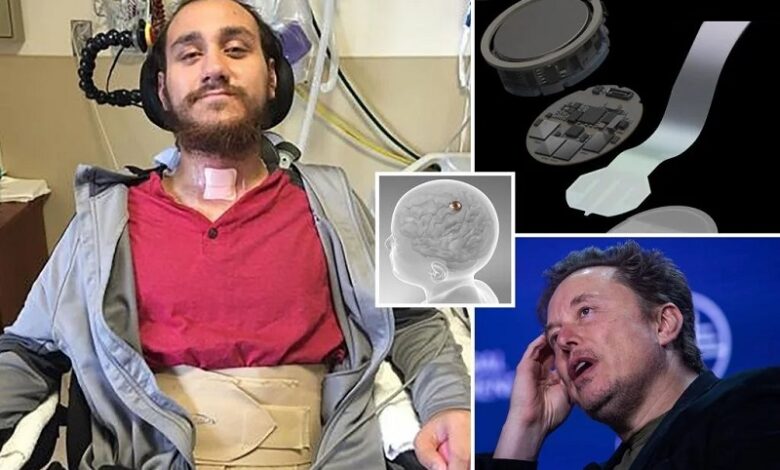
Neuralink, the brainchild of Elon Musk, has been at the forefront of revolutionary advancements in neurotechnology. The company’s latest endeavor, the brain-chip implant known as the “Link” device, has recently encountered a setback. In a progress update shared in a blog post, Neuralink revealed that several threads of the device had retracted from the brain of its first human patient, resulting in diminished effectiveness.
The Link device represents a remarkable fusion of neuroscience and engineering, allowing individuals to control a computer cursor with their thoughts. Comprising over 1,000 electrodes and 64 threads, each finer than a strand of human hair, this cutting-edge technology holds immense promise for individuals with paralysis or other neurological conditions. However, the recent issue with thread retraction highlighted the challenges inherent in such pioneering endeavors.
Neuralink employs a metric called bits per second (BPS) to gauge the speed and accuracy of cursor control. A higher BPS score indicates stronger control over the cursor. The company acknowledged that the thread retraction had compromised the efficacy of the electrodes within the device, leading to a decline in performance. Nevertheless, Neuralink swiftly implemented adjustments, resulting in a significant and sustained improvement in BPS. This progress surpassed the initial performance levels observed in the patient, offering hope for further advancements.
The first recipient of Neuralink’s implant, Noland Arbaugh, captured the world’s attention when he underwent the groundbreaking procedure in January. Paralyzed from the shoulders down following a diving accident in 2016, Arbaugh became the pioneer in Neuralink’s quest to merge technology with the human brain. Despite the setback caused by thread retraction, Arbaugh’s experience with the implant has been transformative.
Reports surfaced suggesting that Neuralink contemplated removing the implant entirely from the patient. However, Musk remained optimistic, expressing confidence in the progress made during a livestream event. He reassured stakeholders that Arbaugh appeared to have fully recovered, underscoring the resilience of both the patient and the technology.
Musk’s tweet celebrating 100 successful days since the implant further underscored Neuralink’s commitment to pushing the boundaries of innovation. Despite the challenges encountered along the way, the company remained steadfast in its mission to revolutionize neurotechnology. The ambitious goal of implanting devices in ten additional human patients by year’s end signaledNeuralink’s determination to expand its reach and impact.
Arbaugh, in a meeting at Neuralink, shared insights into his journey from applying for the human trials to undergoing the implant procedure. Remarkably, the entire process, from application to surgery, took a mere five months, with the surgical intervention lasting less than two hours. Arbaugh’s testimonial highlighted the transformative potential of Neuralink’s technology, allowing him to engage in activities previously beyond his reach, from playing video games to posting on social media and playing chess.
Despite Neuralink’s monumental achievements, questions lingered regarding the long-term viability and safety of brain-chip implants. As the field of neurotechnology continues to evolve, ethical considerations and regulatory frameworks must accompany technological advancements. Neuralink’s willingness to address challenges head-on and collaborate with stakeholders underscores its commitment to responsible innovation.
Neuralink’s journey with brain-chip implants exemplifies the intersection of human ingenuity and scientific discovery. While setbacks are inevitable in the pursuit of groundbreaking innovation, Neuralink’s perseverance and determination continue to drive progress in the field of neurotechnology. As the company navigates the complexities of human trials and regulatory approval processes, its vision of enhancing human capabilities through seamless integration with technology remains unwavering.






