The Unveiling of Glutamatergic Astrocytes: A Potential Game-Changer in Neuroscience and Neurodegenerative Disease Research
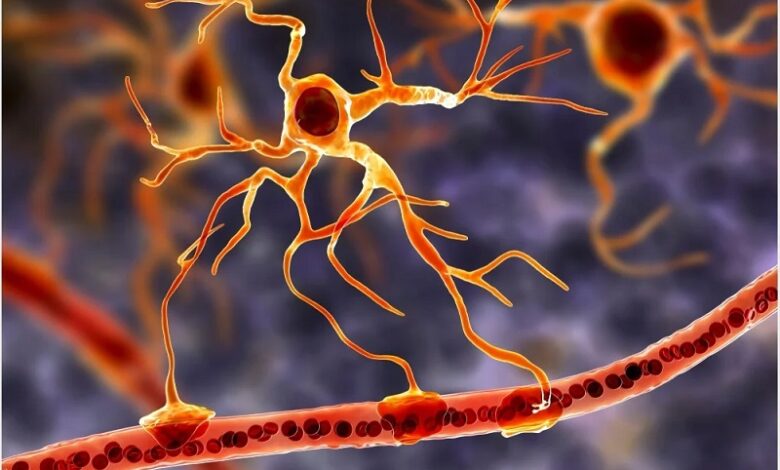
For decades, the field of neuroscience has primarily revolved around the central role of neurons and glial cells in the functioning of the nervous system, including the brain. However, a groundbreaking discovery has emerged, revealing the existence of a previously hidden “hybrid cell” that blurs the lines between these two categories. This revelation has the potential to revolutionize the way scientists approach the development of treatments for neurodegenerative diseases such as Alzheimer’s disease, offering new hope and avenues for research.
Traditionally, neuroscientists have understood the brain’s operations to be predominantly driven by neurons, which are responsible for processing and transmitting information throughout intricate neural networks. These neurons receive crucial support from glial cells, which serve various functions, including providing structural support, energy, and immune support. Among the glial cells, astrocytes, a particular subtype, have been a subject of interest for their proximity to synapses, the critical junctions where neurons connect and communicate. Speculation has long persisted that astrocytes might play a pivotal role in synaptic transmission and, by extension, information processing. However, prior studies have yielded inconclusive results, leaving this theory in doubt.
In a remarkable turn of events, a recent study published in the prestigious journal Nature has the potential to lay these doubts to rest by confirming that astrocytes, akin to neurons, are capable of releasing neurotransmitters. This groundbreaking research employed molecular biology techniques to meticulously examine the contents of astrocytes in mouse tissues. Within these cells, the researchers discovered the presence of the necessary “machinery” for the rapid secretion of glutamate, one of the primary neurotransmitters employed by neurons. However, mere machinery was not enough; the crucial test was whether these machines operated efficiently.
To validate their hypothesis, researchers needed to establish that these “hybrid” cells could release glutamate at a pace comparable to synaptic transmission. To achieve this, advanced imaging techniques were employed to visualize glutamate release from brain tissue within live mice. Astonishingly, this hypothesis was substantiated, marking a groundbreaking moment in neuroscience. Intriguingly, when the glutamatergic astrocytes were disrupted, the research team observed consequences for memory consolidation. Moreover, this disruption revealed potential links to conditions such as epilepsy, exacerbating seizures.
The implications of this study are far-reaching and have the potential to reshape our understanding of brain disorders. Ludovic Telley, co-director of the study and an assistant professor at the University of Lausanne, remarked, “Our discovery reveals an even larger complexity of brain cells than understood until today, a complexity extending also to non-neuronal cells like astrocytes, which enlarges the spectrum of players in brain computations, and at the same time defines more specialized roles for each subpopulation, and therefore increases the number of possible therapeutic targets and refines the therapeutic strategies.” Essentially, this newfound understanding of previously unknown cells broadens the landscape of neuroscience, increasing the complexity of the brain and the nervous system as scientists comprehend it.
Perhaps the most promising aspect of this discovery is its potential to significantly impact the development of treatments for neurodegenerative diseases such as Parkinson’s and Alzheimer’s. Researchers intend to initially explore the protective roles that these novel cells might play in combating diseases like Alzheimer’s. Telley explained, “The first step will be to define if the population of glutamatergic astrocytes is altered in patients (for example, in Alzheimer’s disease patients): this is doable using bioinformatic analysis of existing transcriptomic databases of patients. Once established a role in specific pathologies, we can generate therapeutic strategies (mostly involving gene therapy) to selectively target this cell population and increase or decrease its function.”
However, numerous questions remain unanswered, requiring further research efforts. Among these questions are the precise locations of these cells within the brain, their conservation in humans, and their evolutionary significance in human biology. Researchers are now embarking on a journey to map the distribution of these cells while investigating their associations with various diseases. Simultaneously, they seek to unravel the evolutionary mystery surrounding these newfound cells, shedding light on their role in shaping the complexity of the human brain.
News Mania Desk / Agnibeena Ghosh 11th September 2023